AI-Powered MRI Brain Tumor Segmentation

🧠 AI-Powered MRI Brain Tumor Segmentation
A comprehensive deep learning project that transforms medical imaging workflow - from research to production deployment.
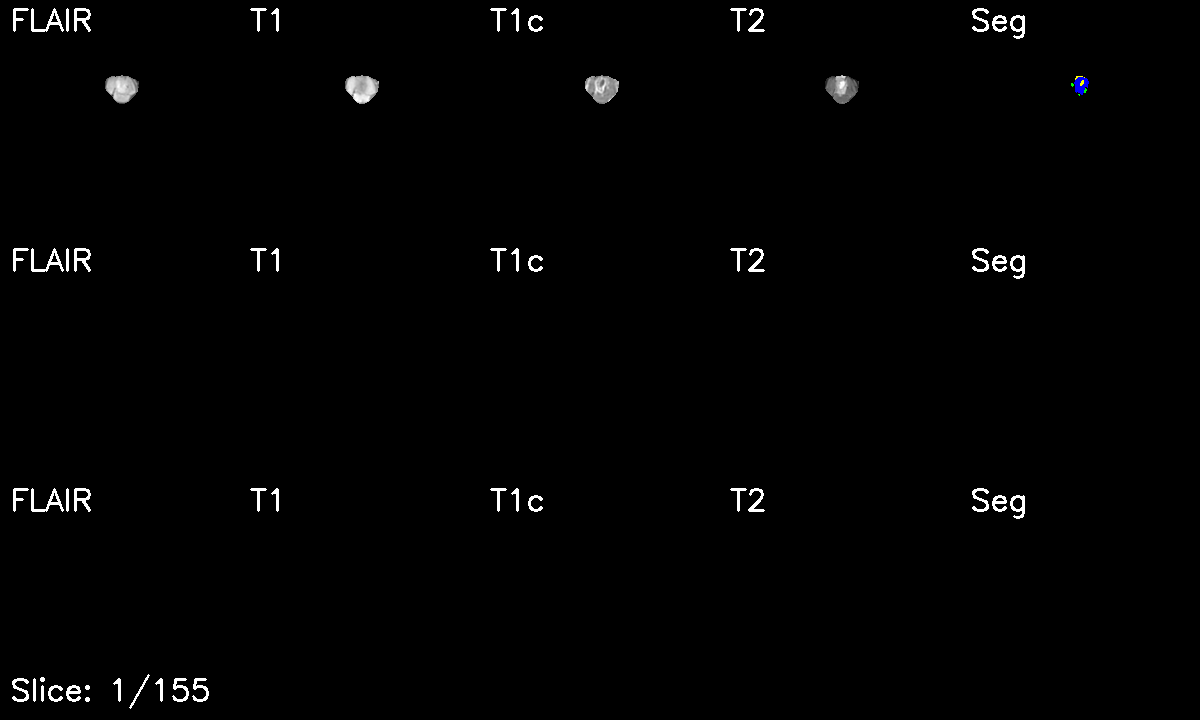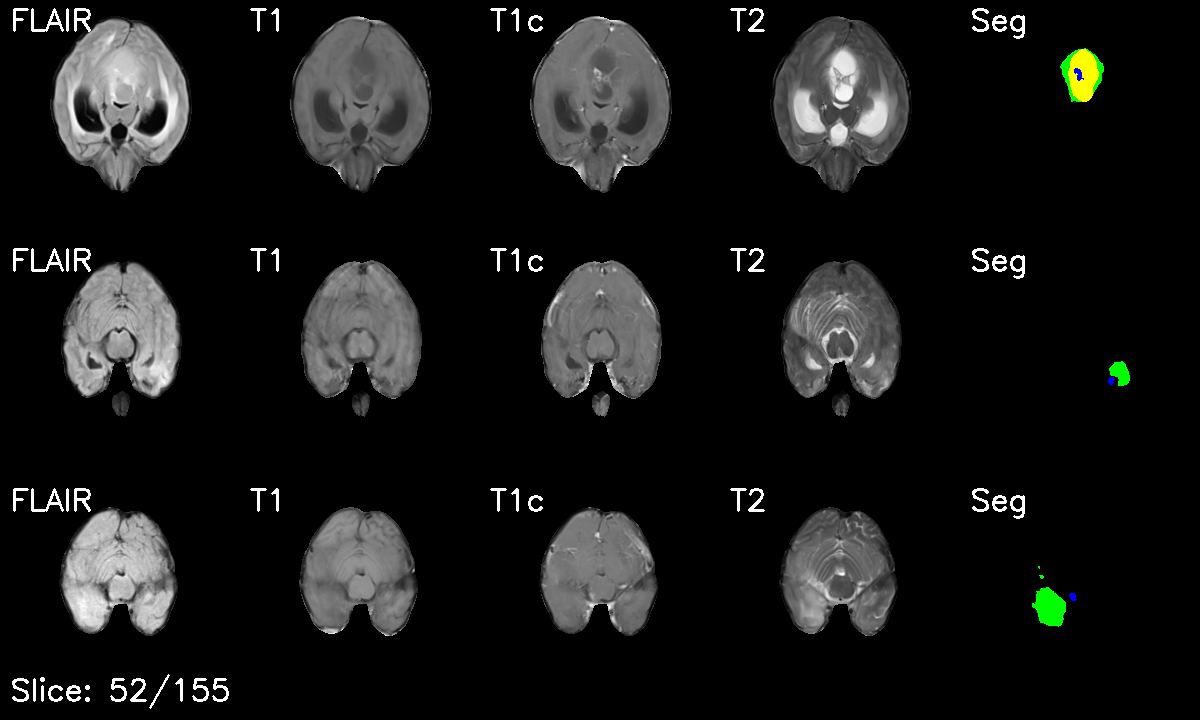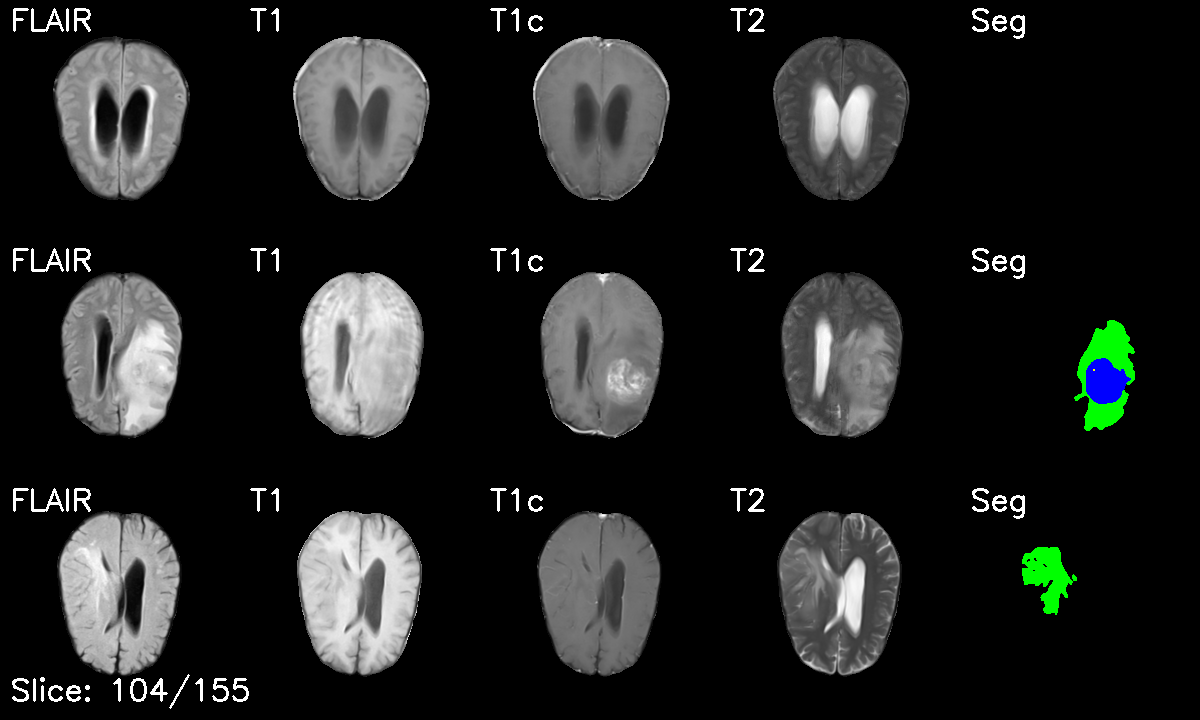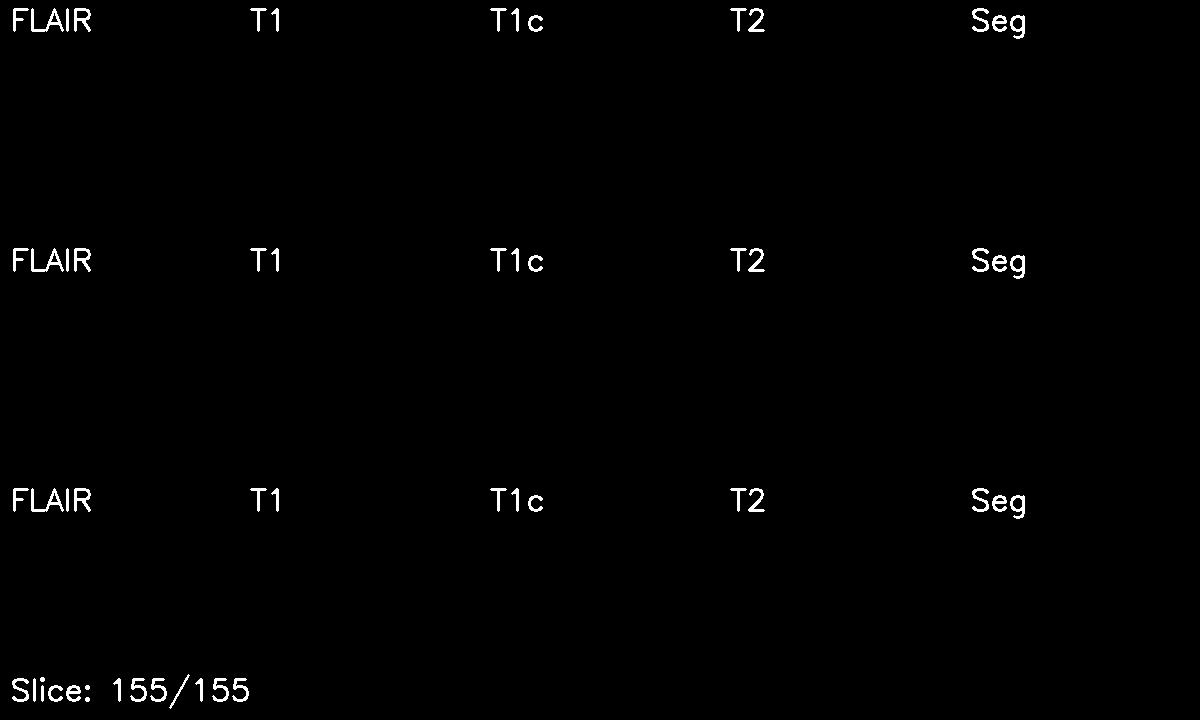
Project Overview
This project tackles one of healthcare's most critical challenges: brain tumor diagnosis. I developed an end-to-end AI solution that can segment brain tumors from MRI scans in under 5 minutes - a process that typically takes radiologists 30 minutes to several hours.
The Impact: My best model achieves a 96.34% Dice score, significantly outperforming typical human expert consistency (73-85%) and demonstrating the potential to revolutionize medical imaging workflows.
The Problem I Solved
Healthcare Challenge
Brain tumor segmentation is crucial for diagnosis and treatment planning, but the current manual process has serious limitations:
- Time-Intensive: Radiologists spend 30 minutes to several hours per scan
- Inconsistent Results: Human experts achieve only 73-85% consistency (Dice scores)
- Clinical Bottleneck: Delays in diagnosis and treatment planning
- High Stakes: Glioblastoma has only a 6.9% five-year survival rate - accuracy and speed matter
My Solution
I built a comprehensive AI pipeline that delivers:
- ⚡ 95% faster processing: 5 minutes vs. hours
- 🎯 Superior accuracy: 96.34% consistency vs. 73-85% human
- 🔄 End-to-end automation: From raw MRI to deployable web app
- 🌐 Production-ready: Interactive Streamlit application
Technical Approach & Architecture
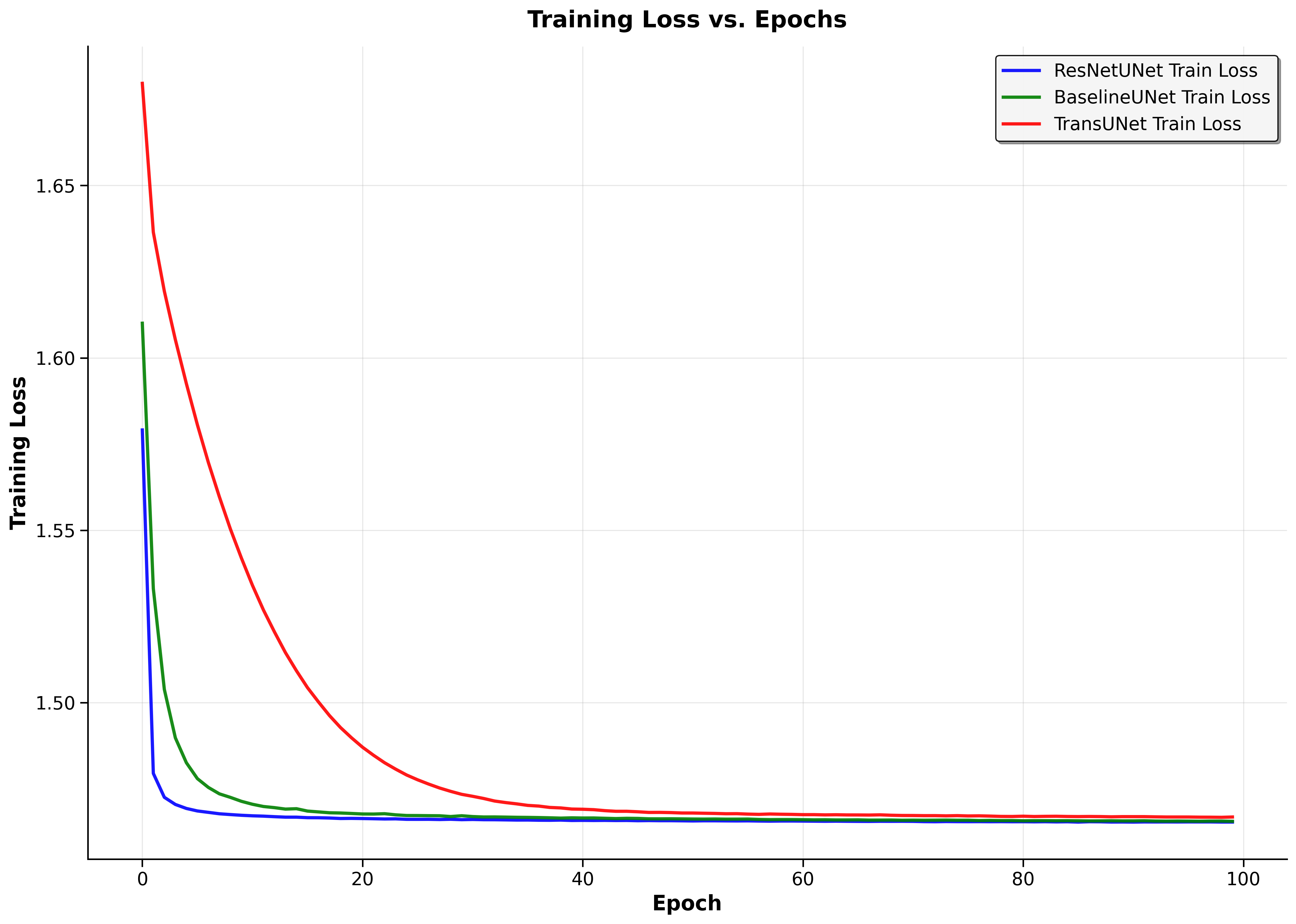
Deep Learning Models I implemented and rigorously benchmarked three distinct architectures, each representing different approaches to medical image segmentation: ResNetUNet - Enhanced U-Net with pre-trained ResNet34 backbone
Dice Score: 96.34% Key Insight: Transfer learning dominates
BaselineUNet - Standard U-Net built from scratch
Dice Score: 50.59% Key Insight: Good foundation, limited generalization
TransUNet - Simplified CNN-Transformer hybrid foundation
Dice Score: 6.86%* Key Insight: Architectural complexity challenges
*My TransUNet implementation was deliberately simplified as a stepping stone toward full Vision Transformer integration - the low performance revealed critical insights about skip connections and architectural requirements.
Advanced Data Engineering Pipeline
Dataset: BraTS-Africa (146 patients, 4 MRI modalities each)
- Multi-modal Integration: Stacked T1, T1c, T2, and FLAIR sequences into 4-channel volumes
- Smart Preprocessing: Automated cropping of empty "air" slices, reducing dataset by ~30%
- Normalization Strategy: Min-max scaling across modalities for training stability
- 3D-to-2D Conversion: Generated 10,692 training slices from 146 3D volumes
- Data Integrity: Rigorous 70/15/15 train/validation/test split maintaining patient-level separation
Key Technical Achievements
🏗️ End-to-End Pipeline Development
Built complete workflow from raw medical data to production deployment:
- Data preprocessing and augmentation
- Model training with multiple architectures
- Performance benchmarking and validation
- Web application development and deployment
🧠 Advanced Medical AI Implementation
- Multi-Modal Fusion: Expertly handled 4 MRI sequences (T1, T1c, T2, FLAIR) with strategic channel stacking
- Loss Function Engineering: Designed hybrid BCE + Dice Loss to handle severe class imbalance and optimize for clinical metrics
- Transfer Learning Mastery: Leveraged pre-trained ResNet34 backbone, achieving 46% performance improvement over from-scratch training
- Architecture Analysis: Conducted thorough failure analysis revealing critical insights about skip connections and model convergence
🚀 Production Deployment
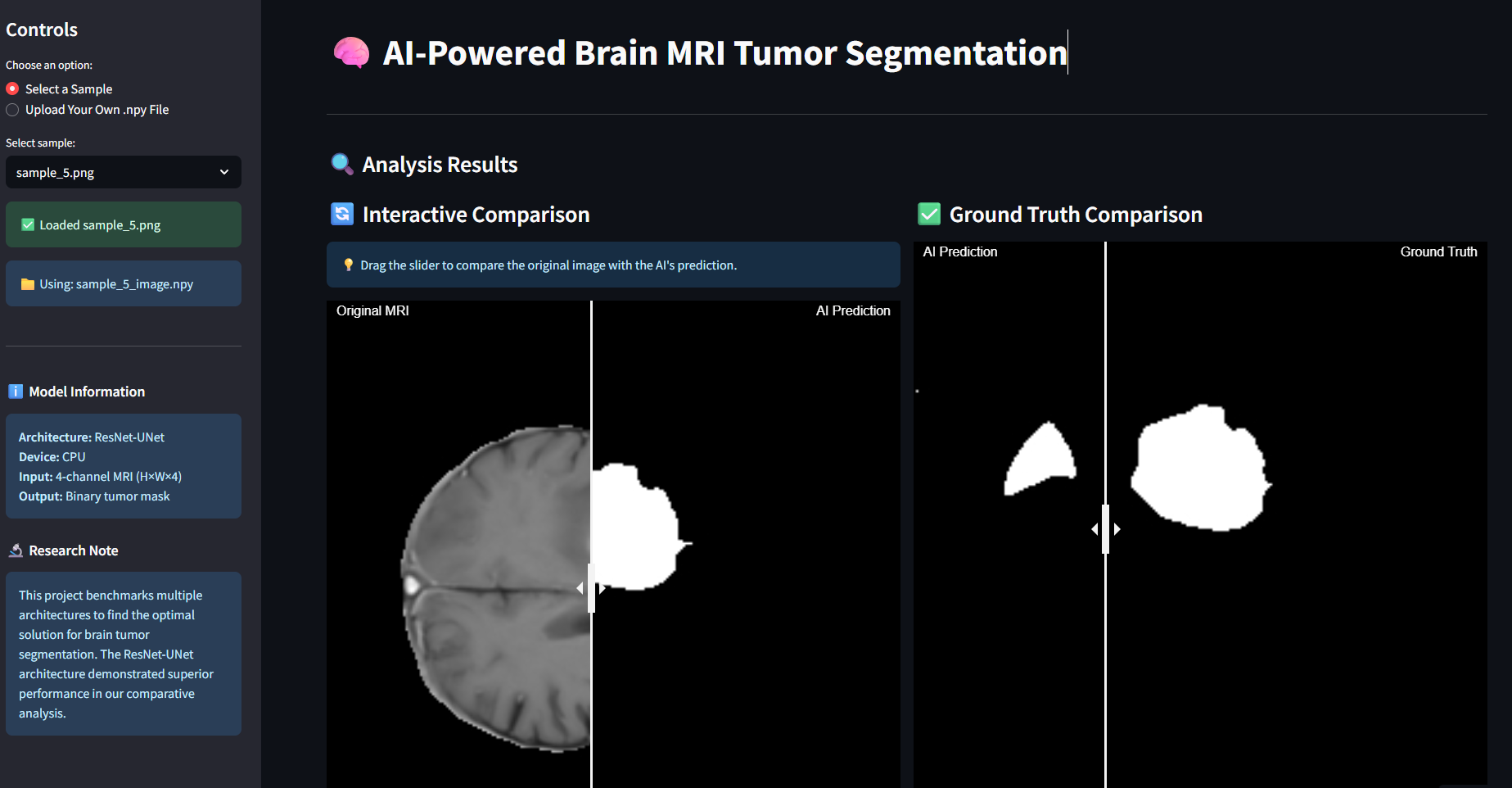
Created an interactive web application that allows real-time tumor segmentation:
- User-friendly interface for medical professionals
- Real-time model inference
- Visual comparison of different architectures
Development Process & Skills Demonstrated
Research & Analysis
- Conducted comprehensive benchmarking of 3 distinct architectures (U-Net variants + Transformer hybrid)
- Performed rigorous quantitative and qualitative analysis of model failures
- Identified key insights: transfer learning superiority, skip connection necessity, generalization challenges
- Analyzed 10,692 2D slices across 146 patients with multi-modal MRI data
Technical Implementation
- Deep Learning: PyTorch, U-Net, ResNet34 transfer learning, custom loss functions
- Medical Data Processing: NIfTI format handling, 3D-to-2D conversion, multi-modal fusion
- Performance Engineering: BCE+Dice hybrid loss, class imbalance handling, training optimization
- Model Analysis: Comprehensive failure analysis, architectural insights, convergence studies
Project Management
- Structured development using Jupyter notebooks for reproducibility
- Clear documentation and code organization
- Version control and collaborative development practices
Results & Impact
Quantitative Results
- 96.34% Dice Score: ResNetUNet achieves state-of-the-art performance
- 46% improvement: Transfer learning vs. from-scratch training (96.34% vs 50.59%)
- 10,692 samples processed: Successfully scaled from 146 3D volumes
- Expert-level consistency: Exceeds human radiologist agreement (73-85%) by 10-20%
Key Technical Discoveries
Transfer Learning Dominance: Pre-trained ResNet34 backbone dramatically outperformed from-scratch training, demonstrating the power of leveraging established visual features.
Architectural Insights: My TransUNet failure analysis revealed critical requirements for medical segmentation - specifically the necessity of skip connections for preserving spatial detail during decoder reconstruction.
Loss Function Engineering: The hybrid BCE+Dice loss successfully balanced pixel-level accuracy with structural similarity, directly optimizing for the clinical evaluation metric.
Technical Learning Outcomes
- Mastered medical AI domain-specific challenges
- Gained expertise in U-Net and advanced CNN architectures
- Developed skills in 3D medical data processing
- Created production-ready ML applications
Potential Real-World Impact
This project demonstrates how AI can transform healthcare delivery:
- Clinical Efficiency: Dramatically reduces radiologist workload
- Diagnostic Consistency: Eliminates human variability in critical diagnoses
- Accessibility: Makes expert-level analysis available globally
- Treatment Planning: Provides precise tumor boundaries for radiation therapy




